Common battery types

There are many types of batteries on the market. They differ from each other in the structure and nature of the chemical processes occurring inside. In light of power outages and the high demand for batteries for the deployment of autonomous power systems, within the framework of the material, we will consider popular types of batteries: automotive, for uninterruptible power supplies and autonomous complexes based on solar panels or wind turbines. Between themselves, these types of batteries are most similar.
Lead acid
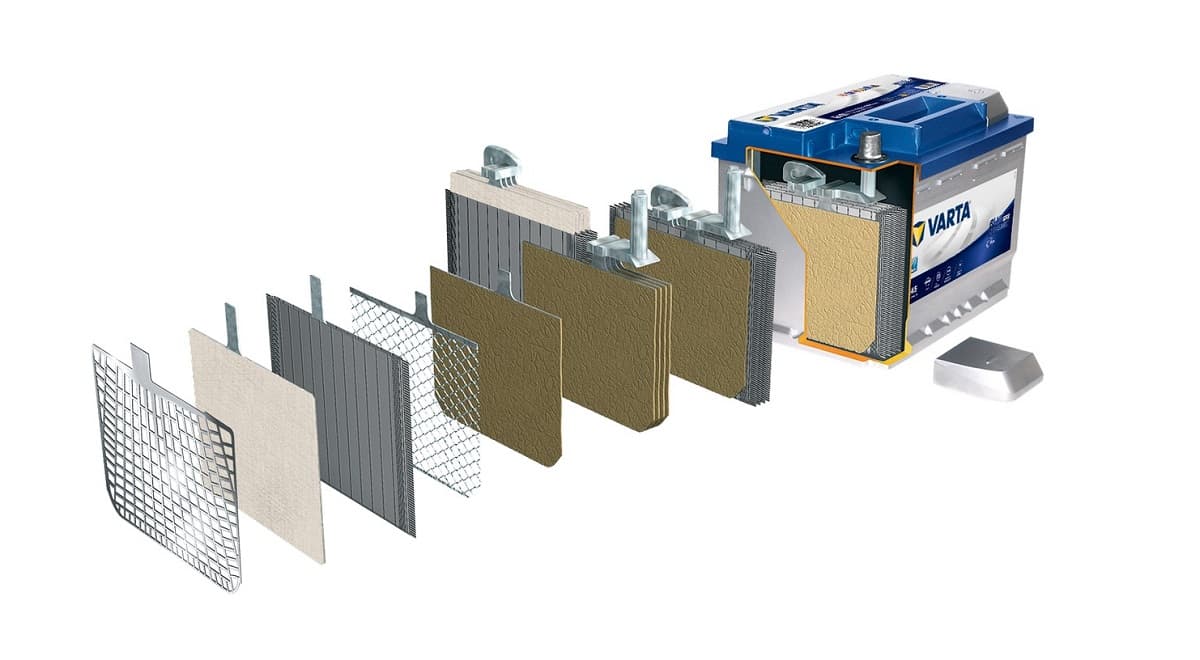
The lead-acid type is the oldest in the context of other common types of batteries. The technology was invented by the French physicist Gaston Plante in 1859. Lead-acid batteries captivate with their versatility and relatively affordable cost. They are used in various types of vehicles, autonomous or backup power supply systems, etc.
Structurally, this type of battery is built in a plastic case and consists of lead plates that are immersed in an electrolyte (an aqueous solution based on sulfuric acid). The principle of operation of a battery is to convert chemical energy into electrical energy when discharging and vice versa - electrical energy into chemical energy when charging. Note that during discharge, lead sulfate deposits form on the plates. And the more the battery is discharged, the thicker the layer of deposits will be. As a result, the battery voltage drops. During battery charging, the process of desulfation occurs, however, in practice, the electrode plates are not completely cleaned and, over time, lead-acid batteries lose their original capacity.
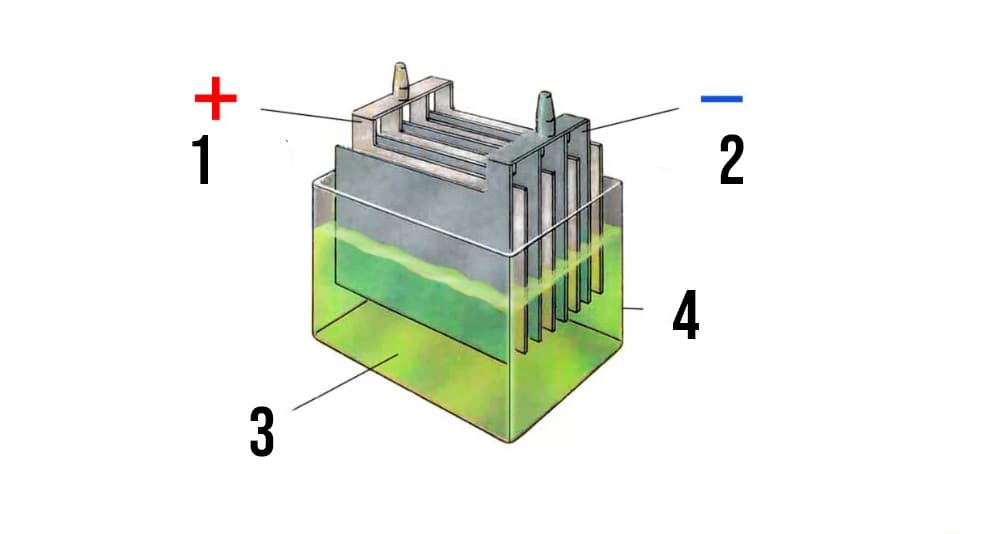 |
| A simplified diagram of the structure of a lead-acid battery: 1 - lead dioxide plates; 2 - lead plates; 3 - liquid electrolyte; 4 - body. |
Lead-acid batteries have low self-discharge, no "memory effect" and can operate over a wide temperature range. At the same time, the less charge remains in such batteries, the less current they produce. Also, lead-acid batteries are afraid of deep discharges, take a long time to charge, have large dimensions and weight. Serviceable models also emit harmful fumes, making them unsuitable for indoor use.
|
Main advantages:
High reliability;
Low self-discharge;
No "memory effect";
Wide operating temperature range;
Affordable cost.
|
Main cons:
Fear of deep discharges;
Emission of harmful vapors;
Long charging time;
Big weight.
|
Lead Acid EFBs
Many of the shortcomings of the original lead-acid batteries are corrected in EFB (Enhanced Flooded Battery) batteries. Their abbreviation stands for "Advanced Wet Battery". In the "gut" of such batteries, thick lead plates are installed without any impurities, moreover, the plate with a positive charge is in a microfiber envelope filled with liquid electrolyte. Microfiber bags prevent shedding of the active mass and significantly slow down the process of plate sulfation during deep battery discharges. As a result, a high efficiency of current transfer is ensured and the risk of short circuits is prevented.
 |
| The lead plates in the EFB battery construction are placed in microfiber bags. |
Due to the use of thicker plates, EFB batteries take longer to replenish their energy reserves. They must be charged with suitable devices, scrupulously controlling the voltage, otherwise the electrolyte may boil and evaporate. For all other points of the programme, advanced lead-acid batteries have only a number of advantages: they are resistant to deep discharges, maintain high productivity in a wide range of temperatures and demonstrate a low level of self-discharge. EFB batteries are more expensive than the original lead-acid counterparts.
|
Main advantages:
High reliability;
Resistant to deep discharges;
Productive work at high and low temperatures;
Low self-discharge.
|
Main cons:
high cost;
Emission of harmful vapors;
Long charging time;
Big weight.
|
Gel GELs
Gel batteries are an even more advanced type of lead-acid batteries with a special thickener in the composition, which brings the electrolyte to a jelly-like state. The gel electrolyte provides maximum contact with the negative and positive plates while maintaining a uniform consistency throughout the volume. Gel batteries are made in a sealed case, they do not emit absolutely no harmful substances in operation and do not require maintenance.
 |
| The electrolyte inside gel batteries is in a jelly-like state. |
Gel batteries bribe with high reliability, environmental friendliness, low self-discharge, long service life. At the same time, they are not the best suited for buffer operation - standing ready for a long time in order to maintain power supply in the standby mode for a short time. It makes sense to purchase such batteries for a UPS when the “uninterruptible” has to turn on almost daily - for example, for unstable networks with constant and long power outages.
|
Main advantages:
High reliability;
Resistant to deep discharges;
Low self-discharge;
No need for maintenance;
Long service life.
|
Main cons:
Sensitivity to charge quality;
Sensitivity to short circuits;
Fear of low temperatures.
|
Absorbed AGM electrolyte
The electrolyte in AGM (Absorbed Glass Mat) batteries is absorbed into porous fibers to give it a jelly-like structure. Such batteries are made in sealed cases and have a reduced electrical resistance, which allows you to give significant currents in short periods of time. AGM batteries are suitable for starting power units in cars and are very often used in power backup systems.
 |
| The electrolyte in AGM batteries is absorbed by finely porous glass fibers. |
Competitive advantages of AGM batteries include long service life, low maintenance and overall cost, high energy intensity, resistance to shocks and vibrations. However, AGM batteries do not tolerate overcharging well, are sensitive to low temperatures, and are heavy.
|
Main advantages:
Can deliver high currents in a short time;
Tolerance for deep discharges;
Low self-discharge;
fast charging;
Sealed case;
Shock and vibration resistance.
|
Main cons:
Poor overcharge tolerance;
Sensitivity to low temperatures;
Great weight;
High cost.
|
Li-Ion Li-Ion
Lithium-ion Li-Ion batteries are used in modern realities everywhere. They are widely used in household appliances and mobile gadgets, found themselves as power sources in electric vehicles and energy storage devices. Such batteries consist of a positive anode on copper foil and a negative cathode on aluminium foil. Charging and discharging in Li-Ion batteries is associated with the transfer of lithium ions between the electrodes.
Batteries of the Li-Ion type have the highest ratio of capacity relative to the dimensions of the case, which makes it possible to manufacture powerful batteries with minimal dimensions and weight. This type of battery is used in devices with high power consumption or, if necessary, to ensure maximum battery life.
 |
| Large packages of lithium-ion batteries will serve as an excellent solution for powering "gluttonous" energy consumers. |
Batteries of this type have a large reserve for charge / discharge cycles, support an accelerated charging procedure, are distinguished by the complete absence of a "memory effect" and low self-discharge. The downside of Li-Ion batteries is their high fire hazard, susceptibility to degradation over time, and sensitivity to low temperatures. The charging process of lithium-ion batteries is monitored by a special BMS board that controls charging and discharging, analyzes the state of the components, takes temperature, voltage and resistance readings, and balances the currents between the battery components.
|
Main advantages:
High capacity with compact dimensions;
Large reserve for charge / discharge cycles;
fast charging;
Low self-discharge;
The complete absence of the "memory effect".
|
Main cons:
fire hazard;
Rapid capacity loss at low temperatures;
Requires an electronic protection circuit;
susceptibility to aging.
|
Lithium Polymer Li-Pol
The main difference between Li-Pol batteries and Li-Ion batteries is the type of electrolyte used. In lithium-polymer batteries, its role is played by a special polymer with conductive additives. The electrolyte structure can be different: dry, homogeneous in the form of a gel, or with a finely porous polymer matrix. Li-Pol batteries can take flexible forms and often come in a soft shell instead of a hard case.
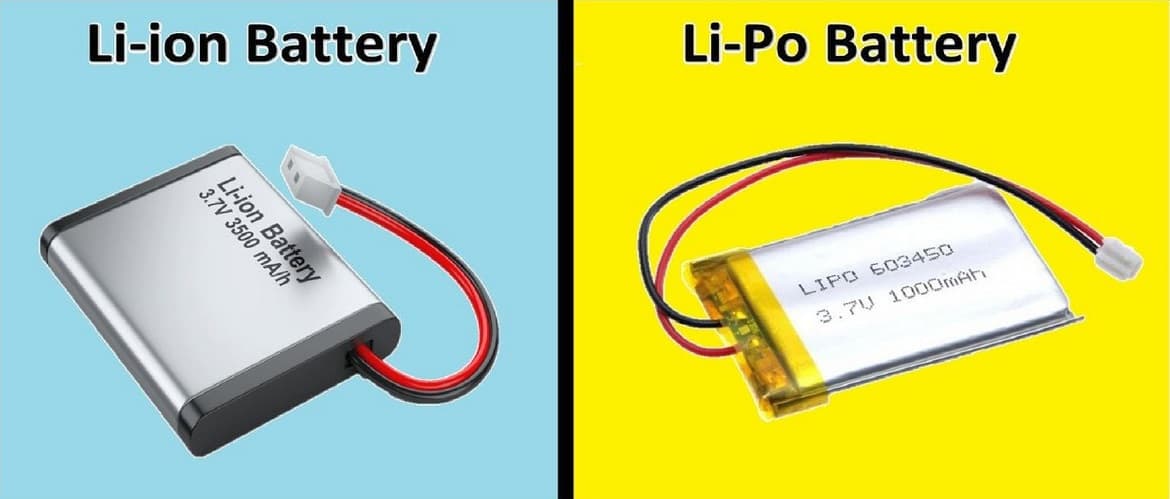 |
| Li-Pol batteries are much "slenderer" than lithium-ion counterparts. |
The positive and negative qualities of lithium-polymer batteries are identical to the Li-Ion type. The only difference is that such batteries can be given absolutely any shape. They are distinguished by grace and thin forms, but they are more expensive than lithium-ion peers.
|
Main advantages:
High specific capacity and energy density;
Voltage stability during the discharge process;
fast charging;
Low self-discharge;
The complete absence of the "memory effect";
Compact dimensions and light weight;
Form variability.
|
Main cons:
fire hazard;
Rapid capacity loss at low temperatures;
Requires an electronic protection circuit;
Degradation during long-term storage.
|
Lithium Iron Phosphate LiFePO4
Lithium-iron-phosphate LiFePO4 batteries are considered the best in terms of parameters for mass consumers. Lithium-titanate batteries are a cut above them, but they also cost an order of magnitude more expensive. LiFePO4 uses lithium ferrophosphate as the cathode material. The main advantages of this type of battery are a large number of charge / discharge cycles (over 2000), chemical and thermal stability, the ability to work without problems in cold weather, shorter charge time (including high currents) and increased operational safety.
 |
| LiFePO4 type batteries are the most advanced to date. |
The control and management of these batteries is entrusted to the BMS board, which guarantees safe voltage and current limits. LiFePO4 batteries have the least chance of thermal runaway and fire. Their working voltage is reduced. However, this has its advantages: it leads to less internal resistance and an increased charge / discharge rate. The only thing such batteries are afraid of is direct exposure to moisture - when interacting with water, active lithium is lost and the energy density decreases. Also, LiFePO4 batteries are larger in comparison with lithium “classmates” (by about 30%), and they cannot be charged at low temperatures. Such batteries are rarely used as starting batteries for cars, since the BMS control board, sharpened for high starting currents, is very expensive.
|
Main advantages:
A huge number of charge / discharge cycles;
Immunity to deep discharges;
Voltage stability;
Fast charging with high currents;
There is no pronounced "memory effect";
Wide operating temperature range;
Durability;
High protection.
|
Main cons:
Low voltage rating;
Sensitivity to direct exposure to moisture;
Cannot be charged at low temperatures;
Larger dimensions compared to lithium batteries.
|
_____
To make the right choice in favor of energy storage devices, you need to correctly prioritize and take into account the features of the further use of batteries. Choosing a battery by type is not a difficult task if you approach the issue wisely and weigh the pros and cons for each common technology.
Articles, reviews, useful tips
All materials































